39 fda approved drug labels
Pharmacogenetic Labeling of FDA-Approved Drugs - PMC Finally, cross-labeling policy issues around implications of updating FDA-approved drug labels on FDA-cleared in vitro diagnostics raised unique challenges. Although not without their challenges, the early experiences with warfarin and other labeling updates were very important in socializing pharmacogenetic principles throughout the FDA's ... Labeling Information | Drug Products | FDA For prescription drug labeling resources (e.g., Prescribing Information, FDA-approved patient labeling, and carton and container labeling), please see the Prescription Drug Labeling Resources web ...
Drug Safety-related Labeling Changes (SrLC) Database Labeling for generic drugs regulated under ANDAs. Labeling for FDA-approved prescription products regulated by the Center for Biologics Evaluation and Research (for example, vaccines, allergenic ...

Fda approved drug labels
Drug Labels | FDA Drug Labels. This is a partial collection of labeling submitted to the FDA Center for Veterinary Medicine (FDA CVM) by animal drug sponsors for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and ... Types of FDA Drug Labeling and Their Requirements - PDG FDA's Guidance for Industry entitled "Help-Seeking" and Other Disease Awareness Communications by or on Behalf of Drug and Device Firms (January 2004) describes two types of drug labeling: FDA-approved labeling, and promotional labeling. An example of FDA-approved labeling is the Professional Package Insert (PPI). Animal Drug Safety-Related Labeling Changes | FDA The labeling for an approved animal drug, such as its carton or package insert, might not reflect the changes for a year or more in the marketplace as the drug company distributes its inventory of ...
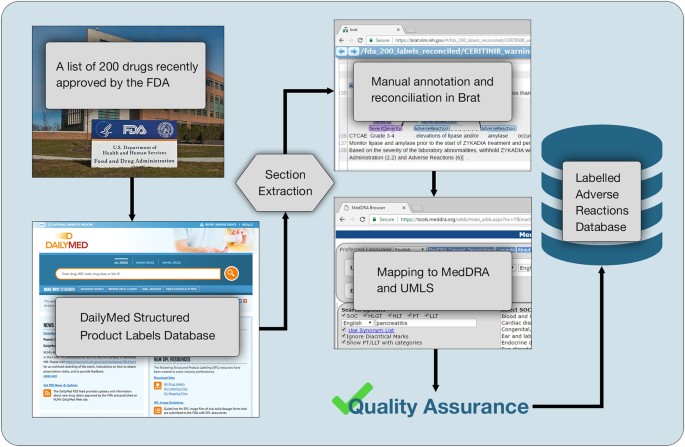
Fda approved drug labels. Pharmacogenomic Biomarkers in US FDA-Approved Drug Labels (2000-2020) Drug labels containing PGx information were obtained from Drugs@FDA and guidelines from PharmGKB were used to compare the actionability of PGx information in drug labels across therapeutic areas. The annual proportion of new drug approvals with PGx labeling has increased by nearly threefold from 10.3% (n = 3) in 2000 to 28.2% (n = 11) in 2020 ... Drug Labeling Overview - Food and Drug Administration The drug labeling provided in this API may not be the labeling on currently distributed products or identical to the labeling that is approved. Most OTC drugs are not reviewed and approved by FDA; however, they may be marketed if they comply with applicable regulations and policies described in monographs. DailyMed The National Library of Medicine (NLM)'s DailyMed searchable database provides the most recent labeling submitted to the Food and Drug Administration (FDA) by companies and currently in use (i.e., "in use" labeling). DailyMed contains labeling for prescription and nonprescription drugs for human and animal use, and for additional products such as medical gases, devices, cosmetics, dietary ... FDA's Labeling Resources for Human Prescription Drugs | FDA The current labeling (also referred to as the in use labeling) submitted by companies to the FDA (e.g., labeling that appears on DailyMed and FDALabel) may differ from the last FDA-approved ...
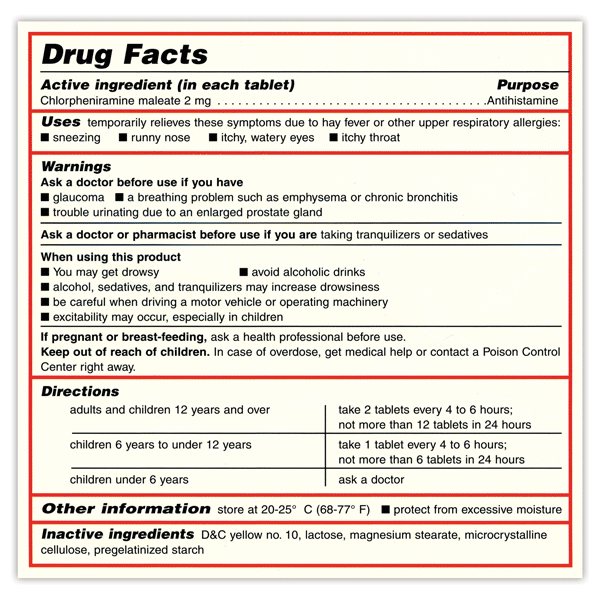
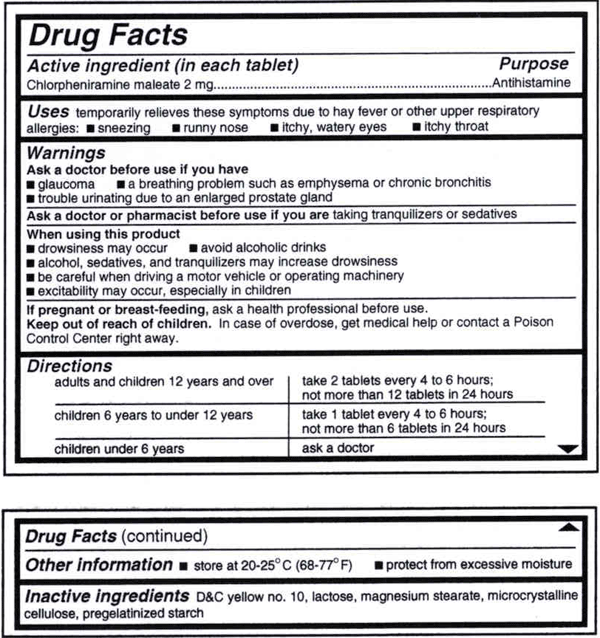
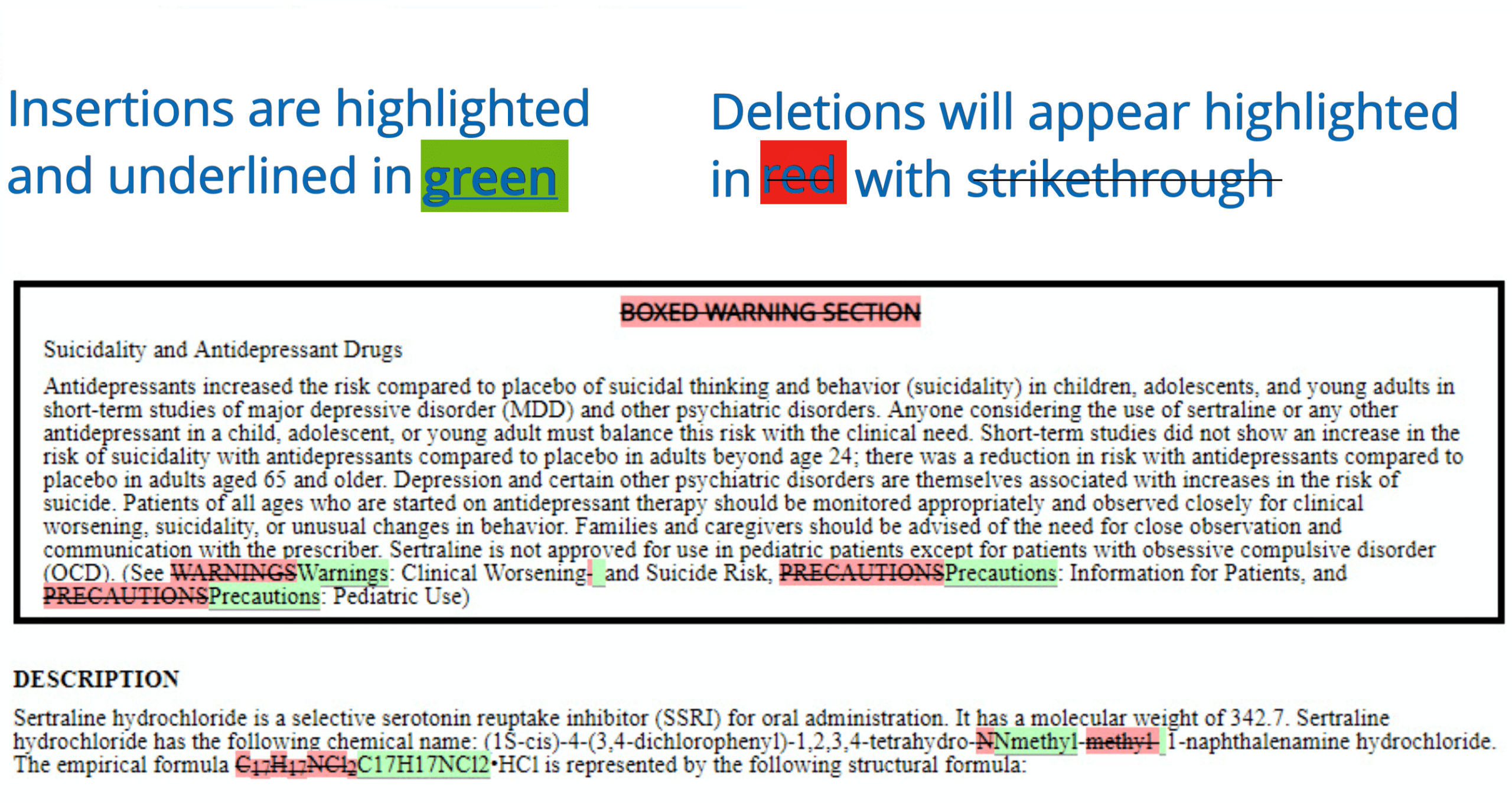
PDF TOPAMAX (topiramate) Label - Food and Drug Administration Suicidal behavior and ideation: antiepileptic drugs increase the risk of suicidal behavior or ideation (5.5) Cognitive/neuropsychiatric adverse reactions: use caution when operating machinery including cars; depression and mood problems may occur (5.6) Fetal Toxicity: use during pregnancy can cause cleft lip and/or palate and Findings on In Vitro Transporter-Mediated Drug Interactions and Their ... Understanding possible follow-up actions on in vitro findings helps determine the necessity of labeling for drug interactions. We analyzed information for in vitro findings on transporter-mediated interactions of drugs approved by the U.S. Food and Drug Administration's Center for Drug Evaluation and Research for the last five years (i.e., 2017-2021) and their follow-up actions for labeling. FDA Label Search - Food and Drug Administration The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor ... TWP's Guide to the FDA-approved Drug Label The FDA-approved "drug label" is a document written by the manufacturer of a particular pharmaceutical drug in collaboration with the U.S. Food and Drug Administration (FDA), and is intended to provide patients and practitioners with key information about one or more approved uses of the drug in the United States, its main chemical ...
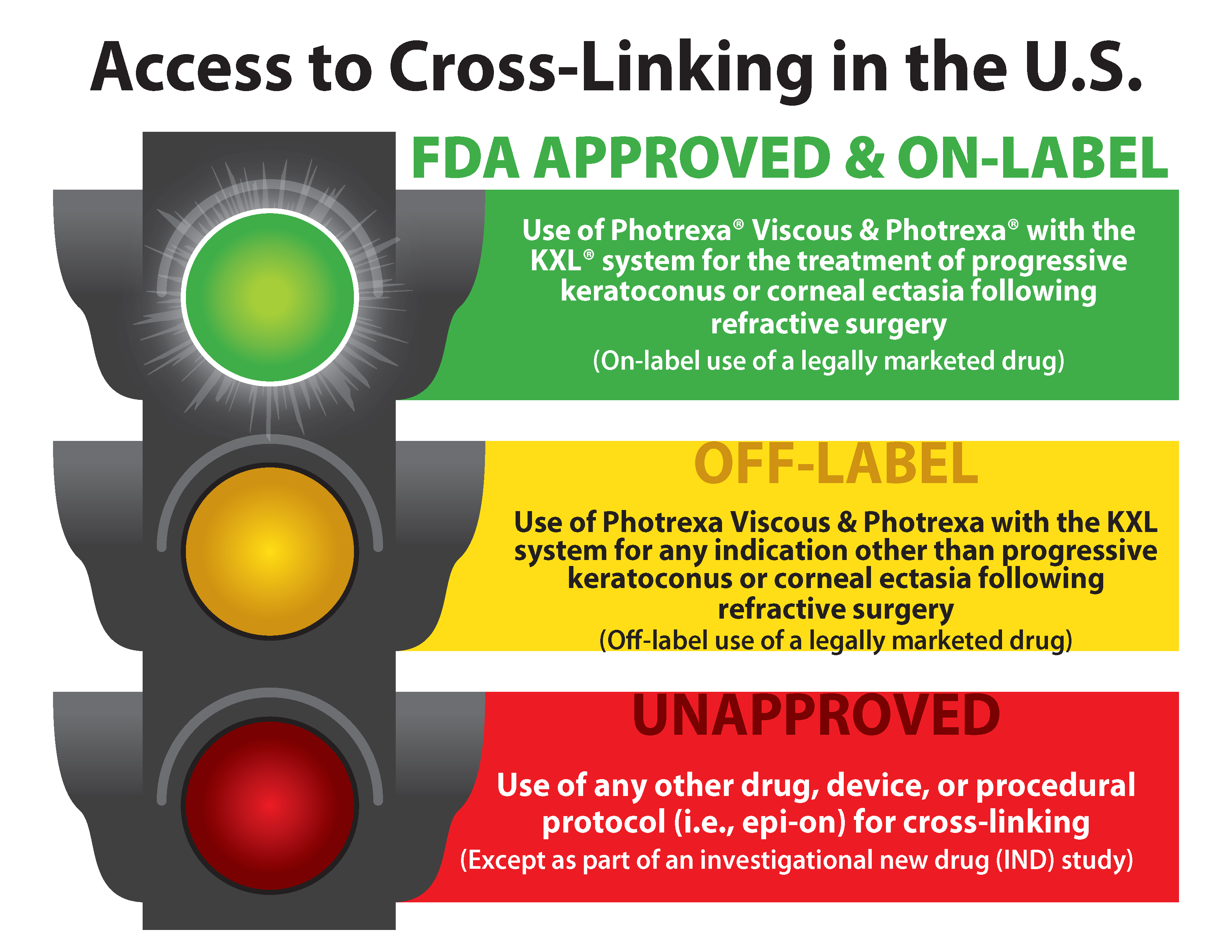
Drug Labels | FDA Drug Labels. This is a partial collection of labeling submitted to the FDA Center for Veterinary Medicine (FDA CVM) by animal drug sponsors for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and medicated feeds. CVM uses the term Blue Bird labels to refer to representative labeling that manufacturers of medicated animal feeds can use as a guide ... FDALabel: Full-Text Search of Drug Product Labeling | FDA FDALabel Database is a web-based application that allows users to perform customizable searches of a database containing over 140,000 labeling documents for FDA-approved drug products, including ... PDF label - Food and Drug Administration 7 DRUG INTERACTIONS . 7.1 Clinically Significant Drug Interactions 7.2 Drugs Having No Clinically Important Interactions with ZOLOFT 7.3 . False-Positive Screening Tests for Benzodiazepines 8 USE IN SPECIFIC POPULATIONS . 8.1 Pregnancy . 8.2 Lactation . 8.4 Pediatric Use 8.5 Geriatric Use . 8.6 Hepatic Impairment . 8.7 Renal Impairment Drugs and Biologicals, Coverage of, for Label and Off-Label Uses Coverage Indications, Limitations, and/or Medical Necessity. Abstract: An off-label/unlabeled use of a drug is defined as a use for a non-FDA approved indication, that is, one that is not listed on the drug's official label/prescribing information. An indication is defined as a diagnosis, illness, injury, syndrome, condition, or other clinical ...
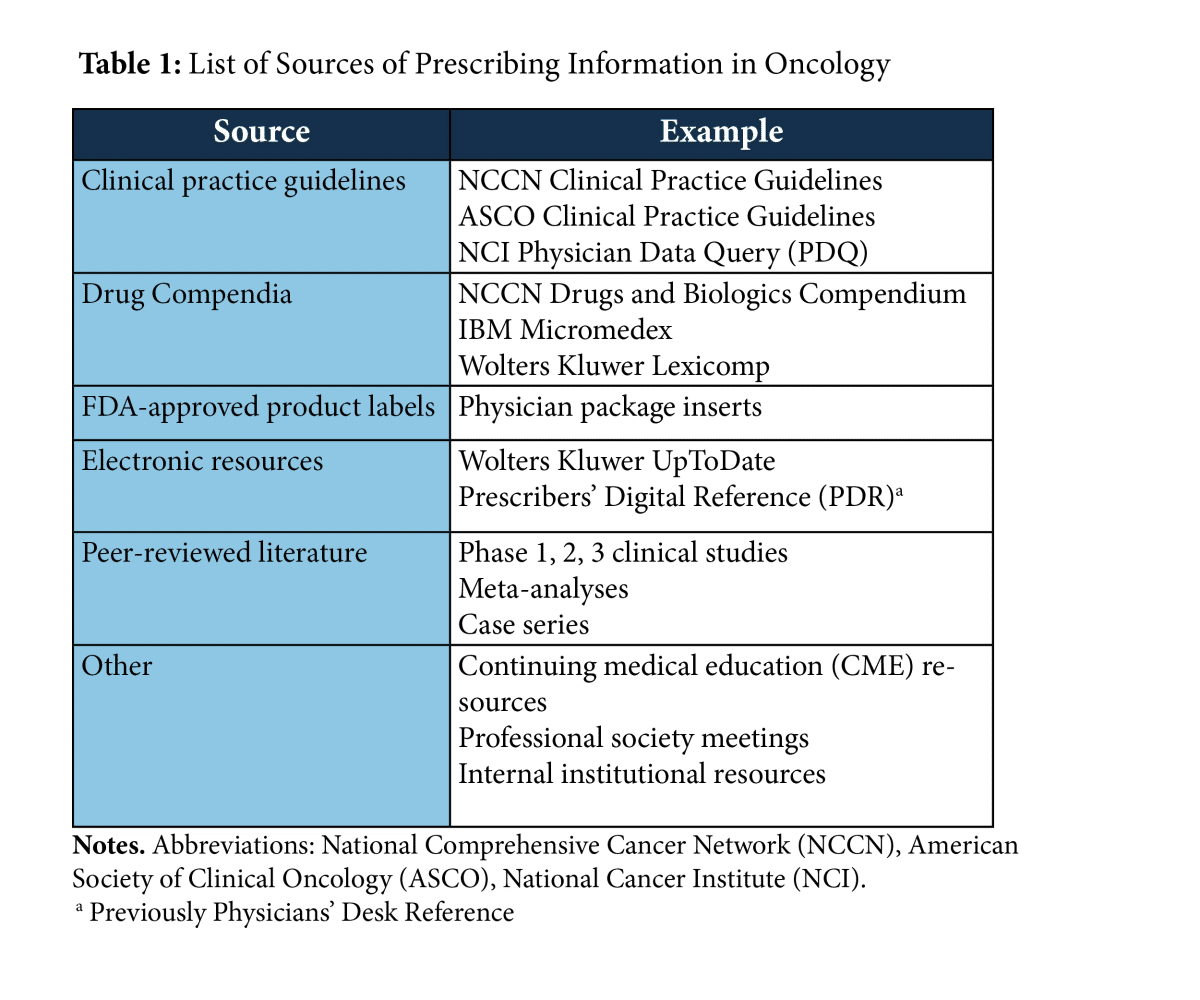
Table of Pharmacogenomic Biomarkers in Drug Labeling | FDA Drug labeling may contain information on genomic biomarkers and can describe: The table below lists therapeutic products from Drugs@FDA with pharmacogenomic information found in the drug labeling ...
PDF Neurontin (gabapentin) Capsules Neurontin (gabapentin) Tablets ... FDA Approved Labeling Text dated 03/01/2011 Page 3 . Elimination: Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing.
Animal Drug Safety-Related Labeling Changes | FDA The labeling for an approved animal drug, such as its carton or package insert, might not reflect the changes for a year or more in the marketplace as the drug company distributes its inventory of ...
Types of FDA Drug Labeling and Their Requirements - PDG FDA's Guidance for Industry entitled "Help-Seeking" and Other Disease Awareness Communications by or on Behalf of Drug and Device Firms (January 2004) describes two types of drug labeling: FDA-approved labeling, and promotional labeling. An example of FDA-approved labeling is the Professional Package Insert (PPI).
Drug Labels | FDA Drug Labels. This is a partial collection of labeling submitted to the FDA Center for Veterinary Medicine (FDA CVM) by animal drug sponsors for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and ...















.jpg)




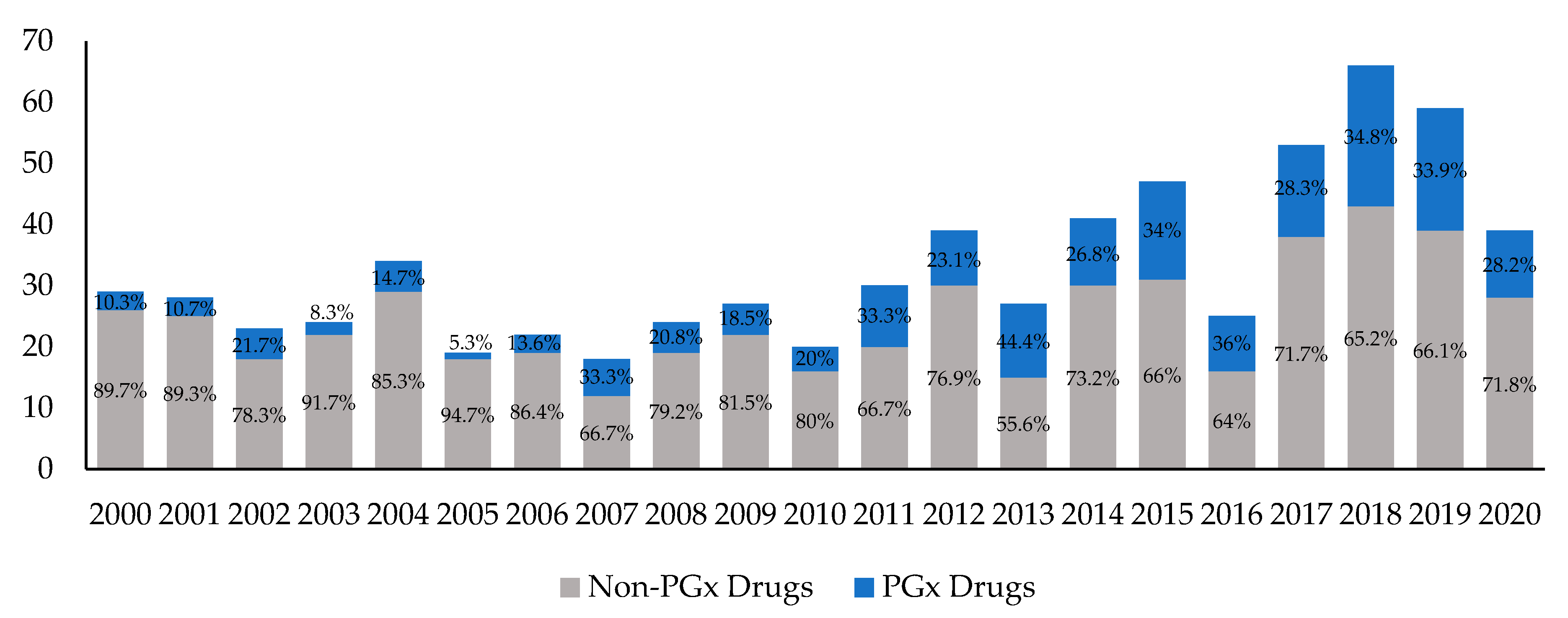


Post a Comment for "39 fda approved drug labels"